Search Product
Structure Search
Search
Advantage Products
Location: Industrial Info
Diabetes drug Victoza was approved to extend label in Europe
2017-08-01
来源:转载自第三方
1 August 2017
Recently, the European Commission updated the labeling of Novo Nordisk's diabetes drug Victoza (liraglutide), which is the only drug that has been certificated to improve blood sugar levels in type 2 diabetes in Europe and also prevent cardiovascular risk.
Diabetes mellitus is a chronic disease, when the pancreas can not produce enough insulin or the body can not effectively use the insulin, diabetes occurs. it will cause serious damage to many systems of the human body without control over time, such as the heart, blood vessels, eyes, kidneys and nerves. The risk of heart attack and stroke increases 2-3 times in adults with diabetes.
GLP-1 is a hormone secreted by small intestinal cells, it can promote insulin secretion, inhibit glucagon secretion, speed up glucose metabolism, delay gastric emptying, and inhibit the appetite. GLP-1 analogues can help patients control blood sugar levels, while suppressing appetite. Similarly, there is a DPP-4 inhibitor, whose hypoglycemic mechanism is similar to that of GLP-1 analogues on in vivo glycemic regulation—inhibition of GLP-1 degradation by inactivating DPP-4, resulting in hypoglycemic effect.
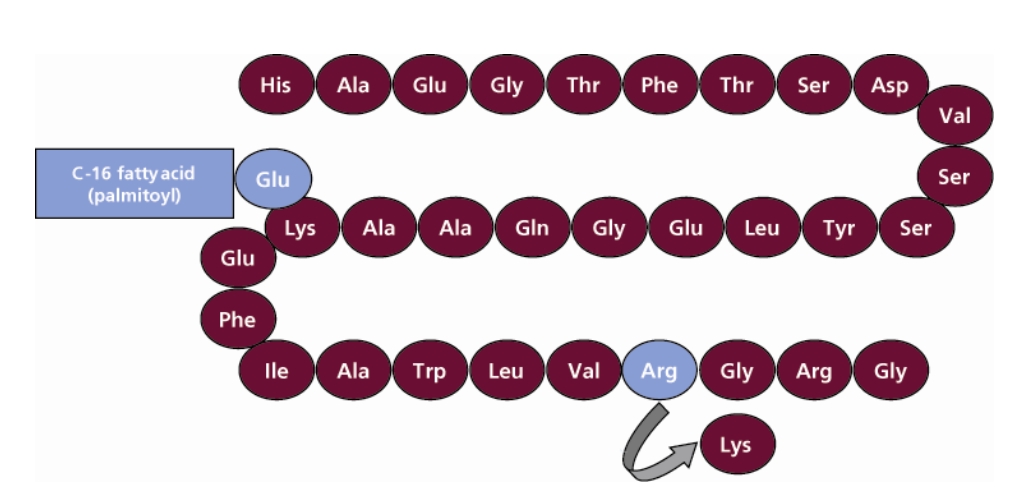
Victoza (liraglutide) is a glucagon-like peptide 1 (GLP-1) receptor agonist. It replaces an amino acid of natural GLP-1 and adds a 16 carbon palmitoyl fatty acid side chain to the molecule, which overcomes its susceptibility to degradation while retaining the efficacy of natural GLP-1. With this change, the drug can achieve a good hypoglycemic effect with only one injection per day, and provide a variety of hypoglycemic benefits. Victoza was approved for listing in the EU in 2009 and approved by FDA in 2010, it is now available for sale in 85 countries.
In June last year, Novo Nordisk announced a positive long-term data of LEADER for Victoza at the 76th American Diabetes Association (ADA) Scientific Conference in New Orleans, USA. Data show that Victoza significantly reduced the risk of composite cardiovascular events (including cardiovascular death, nonfatal myocardial infarction, nonfatal stroke) by 13% compared with placebo, reaching the primary end point of the study. In this study, Victoza's safety was consistent with previous studies. The most common adverse event was gastrointestinal events, and the incidence of pancreatitis in the Victoza treatment group was not significantly decreased compared with the placebo group.
Diabetes will increase the risk of heart disease, stroke and other diseases, and cardiovascular complications in turn will significantly affect the health and expectation of life of patients with diabetes. Therefore, diabetes drugs have been placed in hope to reduce the risk of cardiovascular complications in patients. The European Commission has approved the labeling expansion of Victoza, which will improve the opportunities for patients to use GLP-1 drugs.
Related links: GLP-1 antidiabetic drugs semaglutide will soon be listed in Japan
The Mechanism and Common Drugs of DPP-4 Inhibitors
The Mechanism and Common Drugs of DPP-4 Inhibitors
Edited by Suzhou Yacoo Science Co., Ltd.
如果涉及转载授权,请联系我们。












