Search Product
Structure Search
Search
Advantage Products
Location: Thematic focus
How to judge the quality of the buffer? What features does buffer need?
2019-03-04
来源:亚科官网
The Buffer solution is usually a solution consisting of a "weak acid and its conjugate base" or "weak base and its conjugate acid" buffer pair, which can slow the change of pH when a certain amount of other substances are added. Let's take a look at the specific knowledge about buffers.
What is a buffer?
The buffer is an aqueous solution containing a conjugate acid/base pair. The pH range of the buffer is determined by its pKa value. The pKa value is defined as the pH at which 50% of the molecules are in an acid structure and 50% in a basic structure. A general rule of reference for buffers is that the pH should be within 1 pH unit of the pKa value to ensure good buffering capacity. This ensures that there are enough molecules present in the acid structure and the basic structure to neutralize them when H+ or OH- is added. In this way, the buffer prevents changes in protein stability caused by pH changes.
Good buffers must have the characteristics
1. water soluble
2. chemical stability
3. Good buffering capacity in the desired pH range
4. Good compatibility with analytical and experimental application conditions
5. Good compatibility with other solutesMany substances can be used in biological buffers. The most commonly used buffer components typically have near-neutral pKa values and can be used around physiological pH ranges. Table 1 lists the four most commonly used biological buffers, their respective pH application ranges, and their respective advantages and disadvantages that may affect the protein purification process. To ensure adequate buffering capacity, the concentration of these buffers is typically 25 mM.
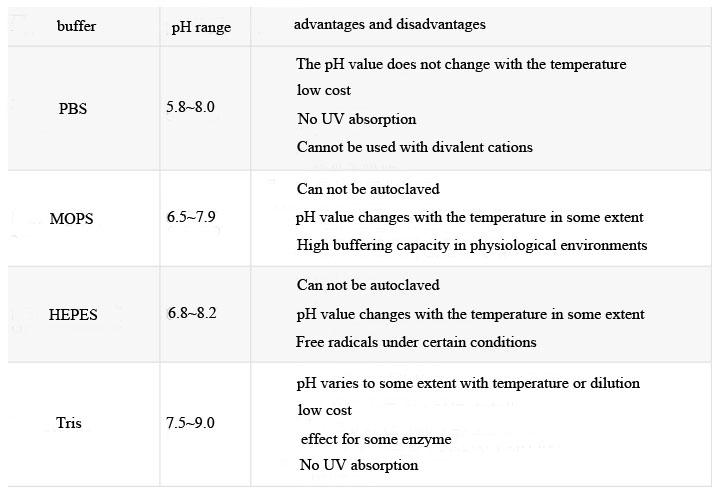
The most commonly used biological buffer for protein purification. Buffers maintain their buffering capacity over a range of pH values, and some buffer components can affect certain chromatographic processes or analytical experiments.
The role of buffer solution
The function of the buffer solution is to adjust the pH value of the solution within a limited range so that the acidity of the solution to be tested conforms to the range specified by the analytical method. For example, phenol can be combined with 4-aminoam in the presence of an alkaline medium and potassium ferricyanide. The tepyrine reaction produces an orange-red antipyrine dye which is most suitable for preventing the interference of aromatic amines at a pH of 10 ± 0.2. To this end, it is necessary to add an ammonia-chlorinated buffer solution to the solution to be tested to adjust and control the pH of the solution to be tested to be 10.0 ± 0.2.
In practice, some analysts do not understand the mechanism of the buffer solution. When the buffer solution prepared and used does not match the specified value, in order to make the buffer solution meet the requirements, it is strong acid or strong acid such as hydrochloric acid or sodium hydroxide. The base is adjusted so that the buffer solution can reach the desired pH as quickly as possible. However, the result is counterproductive. Although the pH of the solution is adjusted, its buffer system is destroyed and the effect is lost. The buffer solution is generally a pair of conjugates composed of a weak acid and a weak acid salt thereof or a weak base and a weak base salt thereof.
Related links: Buffer
Edited by Suzhou Yacoo Science Co., Ltd.












