Search Product
Structure Search
Search
Advantage Products
Location: Industrial Info
CAS:935-79-5| The Application of THPA in lithium-ion batteries
Product Name/English Name:cis-1,2,3,6-Tetrahydrophthalic anhydride
English abbreviation:THPA
CAS:935-79-5
Molecular Formula:C8H8O3
Article No.:S1112
Structural Formula:
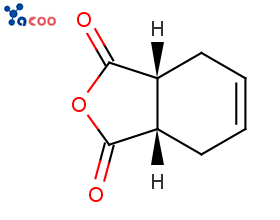
Product Introduction
THPA is an important chemical product, mainly used as a curing agent for epoxy resins, a modifier for unsaturated polyester resins and alkyd resins, and an important intermediate in the synthesis of pesticides and pharmaceuticals. In addition, THPA can also be applied to lithium-ion batteries.
Application of THPA
Lithium ion batteries have become the most promising energy storage device due to their high energy density, long cycle life, low environmental pollution, and good safety performance. With the widespread application and popularization of various intelligent portable devices, mobile terminals, and electric vehicles, the development of new lithium-ion batteries that combine the advantages of high energy density, high power, long service life, and high stability is currently a hot research topic in new energy. LNMO material has the advantages of working voltage of 5V (vs. Li+/Li) and good high-temperature cycling stability, making it one of the most advantageous cathode materials for lithium batteries. However, the compatibility between existing commercial electrolytes and LNMO materials is poor. Once the voltage exceeds 4.2V, the decomposition products of the electrolyte undergo dehydration reaction and LiPF6 reaction to produce HF, leading to the dissolution of Mn and Ni on the LNMO cathode material and the destruction of its spinel Fd structure. In order to improve the high voltage resistance of LNMO lithium-ion battery and have important application value, CN111244544B developed a preparation method of self-assembled 5V nickel manganese acid lithium ion battery cathode interface facial mask functional electrolyte. The electrolyte consists of four parts, specifically:
Electrolyte solvent: ethylene carbonate (EC), dimethyl carbonate (DMC), and methyl vinyl carbonate (EMC) with a volume ratio of 2:3:5;
Mixed lithium salts: including lithium hexafluorophosphate (LiPF6) and lithium difluorosulfonamide (LFSI);
Solvent stabilizer: sulfolane, with an added weight of 15% of the total amount of electrolyte;
Facial mask forming initiator: cis-1,2,3,6-tetrahydrophthalic anhydride, added weight is 1.5% of the total electrolyte;
The electrolyte prepared by the present invention utilizes the characteristic that the polarity of the monobasic anhydride is greater than that of the carbonate solvent, and utilizes the lone pair electrons of the anhydride bond to occupy the empty orbit of the transition metal in the nickel manganese lithium cathode material for complexation reaction, thereby tightly adhering to the surface of the cathode material to form a network like CEI film and locking the transition metal, and exposing the alkyl site to the outside to undergo electrochemical polymerization with some carbonates and lithium salts, The outer layer of the mixed CEI film of polyalkyl lithium and lithium fluoride, which does not have the ability of electronic conduction, is formed, thus the in-situ solid-liquid interface facial mask is successfully constructed on the cathode surface. The CEI film has the function of specifically inhibiting the dissolution of cathode transition metals, and also has high ionic conductivity and electronic insulation performance, effectively preventing the electrolyte from subsequent direct contact with LNMO, and maintaining the stability of LNMO cathode material spinel structure and Li+embedded active sites, Effectively suppress the continuous degradation of electrolyte on the surface of cathode materials, and improve the stability of the entire internal system of the battery.
References
CN111244544B A Preparation method of functional electrolyte for cathode interface facial mask of self-assembled 5V lithium nickel manganate battery.












